2020 Vision to Pandemic Proof our Health and Future
As we turn the page on the challenging year that was, we yearn for brighter days ahead. The prospect of a return to “life as it was” is, however, unlikely to be a near term reality. Indeed, when the virus known as SARS-CoV2 was unleashed upon the world from Wuhan, China, late in 2019, we could not have imagined that our lives would be forever changed. But such it has.
With nearly 82 million individuals having been infected with SARS-CoV2 globally, COVID-19 has claimed 1.8 million lives to date and over 15,500 in Canada alone. The carnage cannot be wholly captured in the tally of lives lost. The indirect costs are immeasurable. The enormous impact of lockdown and quarantine measures on our society and economy will be felt for generations to come. The mental health toll and other indirect effects of COVID-19 on our healthcare systems must be considered an urgent sub-crisis.
Despite the pain and loss, there is reason for hope and even some silver linings. We have learned to appreciate a simpler life, enjoyed more time with family and by necessity adopted new measures and technology to thrive despite the need for distance and restrictions.
Local Matters
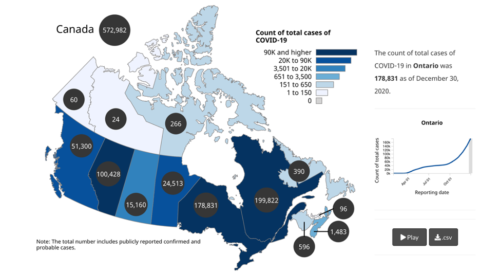
A pandemic anywhere is a problem everywhere. But the dynamics vary greatly depending on local factors. In Canada, the per capita COVID-19 infection and death rates have varied by province due to differences in population demographics as well as varying approaches taken by the provincial governments and varying adoption rates of public health measures.
Figure 1: Count of total cases by province as of December 30th, 2020
Vaccination Nation
As of January 1st, Canada has two approved mRNA COVID-19 vaccines. Despite Health Canada’s authorization and the federal government’s procurement efforts, the process to get vaccine doses into arms has been bogged down by inefficiencies and delays.
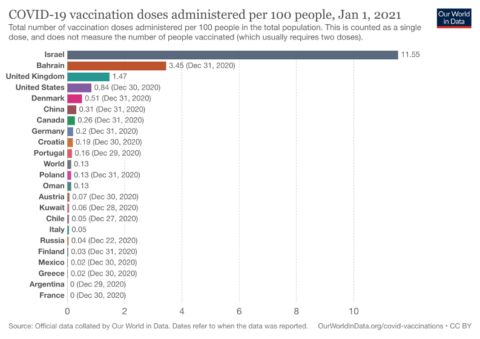
In Israel, vaccination centres are open 24 hours, 7 days a week with no break for holidays or even the Sabbath. Enhanced access and a coordinated distribution and communication strategy have allowed more than 1 in 10 Israelis to have received their first dose of vaccine to date. Canada by comparison, as of December 30th, had vaccinated only 99,559 or 2.6 per 1,000 people.
Figure 2: Cumulative Vaccination Rates per Capita, from December 13th, 2020 to January 1st, 2021
On December 9th, Health Canada authorized Pfizer-BioNTech’s mRNA COVID-19 vaccine. Two weeks later, Canada became the second country in the world after the US to approve a second mRNA vaccine developed by Moderna. The Government of Canada has invested over $1 billion to secure access to approximately 400 million doses of promising vaccines, including 40 million doses of the Moderna vaccine and up to 76 million doses of the Pfizer vaccine.
However, at present, Canada has administered less than 25% of its current supply of approximately 400,000 doses. Over two weeks has elapsed since the first dose was administered in Ontario on December 14th, yet only 29,000 doses have been administered here. At this rate, it is unlikely that most Canadians will have access to a first dose of the vaccine before summer. Even those at highest risk are facing unacceptable delays in accessing vaccines. Every day of delay risks lives.
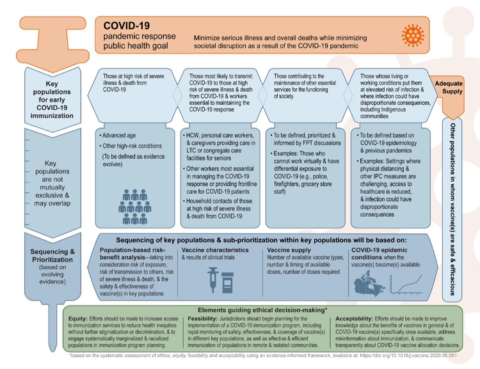
Canada has announced a three tiered prioritization approach to distribute the vaccine based on the NACI guidance (see Figure 3) released in November which identifies key populations for early COVID-19 immunization including; those at high risk for severe illness or death, front-line health care workers and other essential workers, the elderly (starting with those above the age of 80 years) and those living in marginalized communities including the Indigenous population.
Figure 3: NACI guidance on priority populations for immunization
mRNA Vaccines 101
The mRNA vaccines produced by Pfizer-BioNTech and Moderna are the first of a new generation. Messenger RNA technology however is not new. Synthetic single strand genetic code has been developed for therapeutic use over the past thirty years predominantly as a cancer therapy but more recently to vaccinate against RNA viruses. Within a week of Chinese researchers first publishing the genetic sequence of the novel coronavirus, a team of researchers at the University of Pennsylvania began to develop synthetic mRNA to target the virus’ outer shell, the spike protein. Both Moderna and Pfizer licensed this formulation to create vaccine candidates. At unprecedented speed, within 66 days of the sequencing of the SARS-CoV2 genome, Moderna had announced a vaccine and began US clinical trials.
mRNA Vaccines and Immunity
These novel vaccines work by instructing the cell to develop an antibody response in the same way that infection would illicit immunity. The vaccine contains a strand of genetic code (RNA) that has been synthesized to enter the cell, but not the nucleus. The vaccine RNA, which contains the code for viral spike protein, is then translated by the cell into proteins. Vaccine RNA works in the cytoplasm and cannot combine with DNA of the human host genome which is contained within the nucleus. The proteins encoded by the RNA represent only those found on the viral envelope and thus are unlikely to result in symptomatic disease associated with infection, namely COVID-19. Because the spike protein plays a critical role in infecting a host’s lung cell (via the ACE-2 receptor) they are ideal targets for vaccines.
Digging into Vaccine Data
The clinical studies evaluating these mRNA vaccines were designed to show efficacy against symptomatic disease, COVID-19 rather than infection. Symptomatic disease is defined as having recognized symptoms of COVID-19 including respiratory symptoms, fever, or loss of smell or taste AND a positive RT-PCR test confirming the presence of viral RNA. Moderna’s trial looked at outcomes in three groups: those over the age of 65 years, those between the ages of 18 to 65 years with risk factors and those between the age of 18 to 65 years without risk factors. Pfizer-BioNTech’s trials included those aged 16 years or older. Notably, neither vaccine has been studied or is approved for use in children under 16 years or women who may be either pregnant or breast feeding. Both vaccines have shown excellent efficacy of about 95% after a second dose as measured at 7 days by Pfizer and 14 days by Moderna. The Moderna vaccine has been shown to be 100% effective in preventing in severe COVID-19 disease defined by severe symptoms requiring hospitalization and/or resulting in death.
While both of the mRNA vaccines require a second dose, the timing of this second dose differs, as shown below (see Figure 4). The immunity achieved with just one dose has not been confirmed but early indications suggest that it may be as high as 90% at 4 weeks following the first dose. For this reason, with limited vaccine supply governments are grappling with the decision of whether to hold back a second dose (as is presently the protocol in both the US and Canada). Some experts have called for a first dose to be made available to as much of the population as possible.
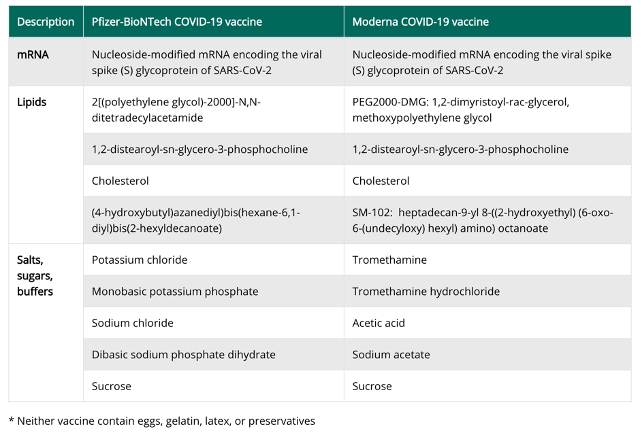
The two mRNA vaccines are developed from the same genetic code but with different formulas (see Figure 5). Adverse reactions have occurred only rarely with either vaccine; rare allergic reactions have been reported with Pfizer’s vaccine and immune reactions have been reported with the Moderna vaccine including tender, enlarged lymph nodes under the arm injected. Those who have had a confirmed infection should defer immunization, until symptoms resolve. Some experts suggest deferring immunization for 90 days from the time of infection or from the time monoclonal antibody therapy or convalescent serum was administered. The vaccine should not be combined or given simultaneously with any other vaccine but rather given at least 2 weeks apart. Presently, it is unclear whether these vaccines will play a therapeutic role in an active infection.
Figure 4: Comparing the approved mRNA COVID Vaccines
| Pfizer-BioNTech | Moderna | |
| Technology | mRNA to spike protein | mRNA to spike protein |
| Dose Schedule | 2 doses at 21 days | 2 doses at 28 days |
| Volume per dose | 30mcg | 100mcg |
| Efficacy | 95%, 7 days after 2nd dose | 94.1%, 14 days after 2nd dose |
| Age Approved | 16 years and older | 18 years and older |
| Storage | -75c, special handling required | -20c or in the fridge for up to 30 days |
Figure 5: Formulation of the two approved mRNA COVID Vaccines
The Prophecy of Herd Immunity
The prospect of vaccine driven herd immunity is attractive, yet far from an achievable certainty. To establish herd immunity, Dr. Fauci and other experts now estimate that 75% to 90% of the population must be rendered immune by either vaccination or infection. As noted above, a portion of the population will not be candidates for vaccination even if available; children, pregnant and lactating women and those with recent confirmed COVID-19. More worrisome, is the risk of vaccine hesitancy limiting rates of vaccination uptake even when supply is available. Accounting for those that do not qualify for vaccination with the anticipated opt outs, it seems very unlikely that we can achieve the high vaccination rates necessary to yield herd immunity.
Other factors stand in the way of a vaccine panacea. While short term efficacy has been shown to be excellent, as measured at 1 and 2 weeks, the durability of this immune response remains unclear. Confirmed cases of COVID-19 reinfection suggests that natural infection does not produce a lasting immune response. Antibody negative serology in individuals with a history of confirmed COVID-19 suggest the absence of a lasting immune response from either infection or immunization. It has been my experience having tested several patients with a history of prior confirmed COVID-19, that the absence of antibodies following established infection is not a rare occurrence. Similarly, we find ourselves susceptible to the common cold year after year due to the lack of a robust and durable immune response to the seasonal endemic coronaviruses that account for common cold.
Escaping the Vaccine
New variants of SARS-CoV2 with dramatically higher rates of transmission (estimated to be from 56% to 70% more infectious than the common variant) have been detected in the UK, South Africa and Nigeria. At least four Canadian cases of the British variant, B.1.1.7, have been identified. A cluster of mutations in the viral genome that affect the spike protein allow for enhanced human to human transmission. It remains unclear whether these variants will prove more virulent leading to more severe disease or whether the mutations will render these variants resistant to the current approved vaccines (both of which target the spike protein).
Plug and Play
Importantly, mRNA technology is very much a plug and play, highly adaptable solution. Now that we have largely established the bench to bedside development, manufacture and distribution strategies required, it should be a relatively easy process to update the “recipe” to target a new more common variant. Scientific officers from both BioNTech and Moderna have confirmed this to be the case despite the unclear implications of the new variants. And for that matter, we are also now more prepared to target any novel RNA virus that should emerge in the future.
Measures still Matter
The limits of vaccination and improbability of herd immunity, mean that the highly effective public health measures of masks, physical distancing and hand washing are here to stay. Vaccines have not yet been shown to prevent infection, but rather symptomatic COVID-19. Asymptomatic transmission is well established. It is likely that an infected individual can harbour and shed virus from their upper respiratory tract (nasopharynx) even in the absence of symptoms. The hope is that while vaccinated individuals may become infected, it is unlikely that they will be infective.
The Mask is the new Condom
In the early 1980s at the start of the AIDS epidemic, began the widespread adoption of the condom into the public sphere as a protective measure to prevent infection by HIV, a blood-borne and sexually transmitted virus. Similarly, the face mask has emerged as the weapon of choice to protect from an airborne pathogen in the COVID-19 pandemic. Just as the condom was here to stay, so too will be the mask. But just as individuals may choose to assume some degree of risk in a sexual relationship and sexually engage without the protection of a condom, we similarly will at a future time choose to engage within a social bubble without a mask.
Some of us of late have felt literally “naked” without our mask, so the analogy of unmasked gathering with unprotected intercourse seems fitting.
As a medical doctor performing procedures requiring close contact with patients, I predict that I will be working with a mask for the remainder of my career (which translates I hope into many decades to come). Just as, having trained in the wake of HIV, I have and will continue to work with gloves and other measures to reduce the likelihood of a needlestick injury and protect from transmission of HIV and any other blood borne pathogen. Indeed, infection control measures are implemented under the assumption that any patient may be a carrier.
The Third Wave
I anticipate that we will see a third distinct wave of COVID emerge in the early spring, before most Canadians with be vaccinated. This third wave could likely be propelled by a viral variant that is more contagious, but hopefully not more deadly. Regardless, masks and other measures should remain highly effective and will remain essential tools to bridge to a time when we can rely on herd immunity or other factors that may be reduce community prevalence of COVID-19. Warmer weather, is likely to be one such factor. By summer, we will be spending more time outdoors and, I hope, enjoying reclaimed freedom reaping the rewards of wintertime quarantine lockdowns.
From Pandemic to Endemic
I have previously suggested that COVID-19 is here to stay as the virus has evolved from a pandemic to one that is endemic. As such, SARS-CoV2 may be containable and controllable but is unlikely to be eradicated. Hence, long term strategies are required to simultaneously address an individual’s risk factors, public health needs, environmental factors, and the changing biology of the virus.
The Long Game
It is likely that we will see an increasing number of post-COVID long-haulers, those suffering from protracted symptoms of COVID-19. Long Covid is estimated to effect 10-20% of infected individuals and commonly women aged 40-60 years who may have experienced a mild acute infection. There are two sub-groups of patients; those who have lasting direct symptoms of viral infection such as shortness of breath and cardiac arrhythmias and those that experience indirect cognitive or psychological sequelae such as fatigue, mental fog and depression. The first group is comprised of those that experience symptoms due to direct viral effects such as scarring on the lungs or inflammation in the heart (both tissues express ACE-2 receptors required by the virus to gain entry to host cells). The second group manifests complex neurological and psychological indirect effects by pathways that have yet to be elucidated. A detailed review article in Scientific American describes this emerging health crisis, that is unfolding within the COVID crisis. What role vaccines will play in preventing or treating long COVID, is presently unknown.
Lest we Forget
Both recent and past history, provides lessons to help shape our future. Not since the Second World War, has the world been brought together to combat a dangerous global threat. But unlike the Third Reich, this threat is invisible.
In the despair following the last global pandemic, the Spanish Flu of 1918, unleashed hatred helped to pave the way for the ascent of Hitler’s extremist, nationalistic and genocidal Nazi party. Indeed, recently published research from the Federal Reserve Bank of New York has shown that the regions of Germany hardest hit by the influenza virus were more likely to vote for the Nazi party.
With another pandemic raging, xenophobia, racism and anti-Semitism are on the rise throughout the world. Let us learn from the past, to ensure dark forces do not usurp the opportunity to expand their influence. Regardless of ethnicity, race or religion, we have all been impacted immeasurably by COVID-19. But disparities and inequities have disproportionately victimized the most vulnerable communities amongst us. Just as the scientific community has come together with unprecedented urgency and unity to develop a new generation of vaccines, so too can the global community work together to extinguish this threat.
Pandemic Proofing our Future
There are a few critical steps that can help pandemic proof our society and our health. Many of these measures require co-ordinated efforts of governments and health officials. Some of these factors, however, are determinants that can be shaped by you. These include simple health hacks that you can implement into your daily lives.
1. Testing and Tracing
Well planned, adequately staffed and funded testing and tracing strategies will be required to ensure a safe more open society. Private-public partnerships will likely be required to cover all places of possible exposure beyond those presently considered essential, such as large workplaces, airports and eventually entertainment venues. Rapid antigen testing which can be self-administered at home has a potential future role, especially in controlling outbreaks or when prevalence of infection is high.
2. Vaccination
While vaccination is a critical pillar in pandemic proofing, it alone will not be sufficient. We will likely have a third vaccine approved in Canada to help facilitate large scale community administration as it does not have the same special handling requirements for frozen vaccines. More than ever, we need public health advocates and clear and coherent messaging to inform the public on vaccine safety and efficacy and ensure their buy in. Even with additional vaccine options and increased uptake, achieving vaccination rates required for herd immunity is an unlikely near-term reality.
3. Public Health Measures
The mask is the new condom. The measures, to some extent, are likely here to stay. Indeed, with efforts to roll out vaccines and an expected third wave to come, it is advised that we double down on the measures. Only with extended measures and enforcement, can we hope to contain the virus and soon emerge from lockdowns into a more stable, secure and prosperous future.
4. Surveillance and Screening
Screening at the border and in the workplace should be required with careful surveillance for infection hotspots, virus variants or outbreaks. New requirements for negative PCR test results within 72 hours for all travellers before gaining entry to Canada is a welcome step. However, delays in implementation (slotted for January 7th), lack of communication and coordination with the airline industry and other loopholes threaten to leave our borders vulnerable.
5. National Stockpiles and Supply Chain
Remember the urge you may have felt early into the pandemic to horde toilet paper? Well, such urgency should be the sentiment driving a co-ordinated effort to create a national stockpile of personal protective equipment and a home-grown supply chain for the manufacture of PPE, critical care drugs and licensed vaccines. A recent investigative article published by the Globe and Mail discusses the flaws and failures of Canada’s public health agency that hindered our pandemic response.
6. Optimized Determinants of Health
Daily life choices can shape our susceptibility to COVID-19 and improve our immune response to vaccination. My top ten tips to boost immunity are described in this recent blog post. Some of these recommendations include: ensuring adequate sleep, getting regular exercise, avoiding excess alcohol and supplementing with vitamin D3. The power to pandemic proof your own health, is within reach.
With 2020 Vision
The road forward will be complicated. The much-anticipated approval of COVID-19 vaccines will not obviate the need for masks and distancing in the foreseeable future. The complex biology of SARS-CoV2 will continue to perplex and challenge us likely requiring further innovation and measures. But, we can learn from the lessons of our recent and distant past. With “2020 vision”, we will be more prepared, secure and proactive in addressing the challenges that are to come.
We have undergone a thorough pandemic pivot at PearlMD. The introduction of a secure and seamless Virtual Health platform has enabled continuity of comprehensive care. Innovations in diagnostics and self-administered testing allow us to determine our patients’ unique biomarkers to shape a plan for improved immunity and their best health. Having completed training on administration of the mRNA COVID vaccine, I am excited to both give and receive it.
The pandemic disruption will necessitate accelerated progress in all industries but especially in healthcare, where it is long overdue. Resilience is not innate but an acquired survival skill honed in trying times like these. Let us move forward into this new year, with resilience and optimism, to pandemic proof our health and our future. May 2021 bring you all happiness, health and bright days ahead.
Sincerely,
Dr. Jennifer Pearlman
Dr. Pearlman is a medical doctor in Toronto, Canada. She is owner and Medical Director of PearlMD Rejuvenation a Precision Integrative Medical Clinic offering expert medical care, leading technology and treatments to help patients achieve Ageless Vitality. Dr. Pearlman is an internationally recognized expert in Women’s health, hormones, aging and cosmetic medicine.